18+ ionic bond calculator
One molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom. Ferrum and atomic number 26.

Ion Beam Science Solved And Unsolved Problems
However if we want to define it more accurately a polar covalent bond is a bond that exists between two atoms consisting of electrons that are unevenly distributed.
. Modification of work by vxlaFlickr. The CED was updated in the summer of 2022 to incorporate the change to the calculator policy for the exam. Scale proportion and quantity.
While Crystal Field Theory explains how orbitals split as ligands approach metal ions VBT. For example an ion looks like this. Valence electrons and ionic compounds.
Must contain at least 4 different symbols. Epoxy is the family of basic components or cured end products of epoxy resinsEpoxy resins also known as polyepoxides are a class of reactive prepolymers and polymers which contain epoxide groups. Water is the chemical substance with chemical formula H 2 O.
Half-life symbol t 12 is the time required for a quantity to reduce to half of its initial valueThe term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable atoms survive. ClO 3 While the chloride ion only contains one element chlorine the chlorate ion contains. Perfectly explained thank u very much.
Structure and properties of. The meniscus appears to be a bit closer to the 22-mL mark than to the 21-mL mark and so a reasonable estimate of the liquids volume would be 216 mL. In general a polar bond is a certain class of a covalent bond.
Because of the higher electronegativity of the oxygen atom the bonds are polar covalent polar bondsThe oxygen atom attracts the shared electrons of the covalent bonds to a significantly greater extent than the hydrogen atoms. Ionic strength is directly proportional to the solubility of the salt. 79 of exam score.
The reaction can be given as. The solution of sodium chromate Na 2 CrO 4 is further purified with sulphuric acid to form a solution from which the crystals of orange coloured sodium dichromate Na 2 Cr 2 O 72H 2 O can be extracted. If an object is moving then it possesses kinetic energy.
The explanation was primarily based on hybridisation concepts. The IUPAC name for an epoxide group is an oxirane. Modification of work by the Italian voiceFlickr.
6 to 30 characters long. I is also dependent on the solubility of the salt in the reaction mixture. Significant Figures Calculator.
C2368 H3183 N1381 O4101 P1832. This can easily be observed in a. 2Na 2 CrO 4 2H Na 2 Cr 2 O 7 2Na H 2 O.
Iron ˈ aɪ ər n is a chemical element with symbol Fe from Latin. Figure 11 Chemical substances and processes are essential for our existence providing sustenance keeping us clean and healthy fabricating electronic devices enabling transportation and much more. What Are Ionic Compounds.
Francis Ezekiel January 18 2020 at 1139 am. Development assistance is delivered through US. Kinetic energy is the energy of motion.
I is the ionic strength. The amount of kinetic energy that it possesses depends on how much mass is moving and how fast the mass is moving.
The term is also used more generally to characterize any type of exponential or rarely non-exponential decay. Epoxy resins may be reacted cross-linked either with. They contain more than one element.
It is a metal that belongs to the first transition series and group 8 of the periodic tableIt is by mass the most common element on Earth right in front of oxygen 321 and 301 respectively forming much of Earths outer and inner coreIt is the fourth most common. Recently Calculated Empirical Formulas. This kind of bond is formed when one atom gains electrons while the other atom loses electrons from its outermost level or orbit.
Has a significant bilateral development assistance program and is also a major donor member to a number of multilateral agencies active in Timor-Leste such as the United Nations Asian Development Bank and World Bank. Nomenclature a collection of rules for naming things is important in science and in many other situationsThis module describes an approach that is used to name simple ionic and molecular compounds such as NaCl CaCO 3 and N 2 O 4The simplest of these are binary compounds those containing only two elements but we will also consider how to name ionic. Oxygen is represented by the Symbol O and Atomic Number 8 is a member of the Chalcogen Group.
The equation is KE 05mv2. 1822 of exam score. Agency for International Development USAID governance health and.
ASCII characters only characters found on a standard US keyboard. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Polyatomic ions are ions with many atoms.
The mixing of orbitals during bond formation was explained by Valence Bond Theory VBT. We can also say that it is the dividing line between the formation of a pure covalent bond and an ionic bond. Cl while a polyatomic ion looks like this.
Modification of work. Students cultivate their understanding of chemistry through inquiry-based lab investigations as they explore the four Big Ideas. IDM Members meetings for 2022 will be held from 12h45 to 14h30A zoom link or venue to be sent out before the time.
Ionic Bond Vs Covalent Bond. Kinetic energy is one of several types of energy that an object can possess. Oxygen is one of the most important elements as it is needed by most forms of life to survive on Earth.
Structure of Water. They are charged just like regular. 4FeCr 2 O 4 8Na 2 CO 3 7O 2 8Na 2 CrO 4 2Fe 2 O 3 8CO 2.
The bond formed by this kind of combination is known as an ionic bond or electrovalent bond. Water is a tasteless odorless liquid at ambient temperature and pressureLiquid water has weak absorption bands at wavelengths of around 750 nm which cause it to appear to have a blue colour. Changing the ionic strength manipulates the solvation of the reactants and intermediates thus changing ΔS and affecting the reaction rate.
Difference Between Baking Soda And Baking Powder. Refer to the illustration in Figure 126The bottom of the meniscus in this case clearly lies between the 21 and 22 markings meaning the liquid volume is certainly greater than 21 mL but less than 22 mL. Electronegativity and Ionic Bonding.
AP Chemistry is an introductory college-level chemistry course. The epoxide functional group is also collectively called epoxy.

Lewis Dot Structure Worksheet Teaching Resources Tpt

Computational Modeling Of Supramolecular Metallo Organic Cages Challenges And Opportunities Acs Catalysis

Pgnminadi8 Q M

Elastic Scattering Of Electrons From The Ions Of Argon Isonuclear Series Iopscience

C8h18 O2 Co2 H2o Chemical Equation Balancer

Octet Rule Detailed Explanation With Examples Exceptions
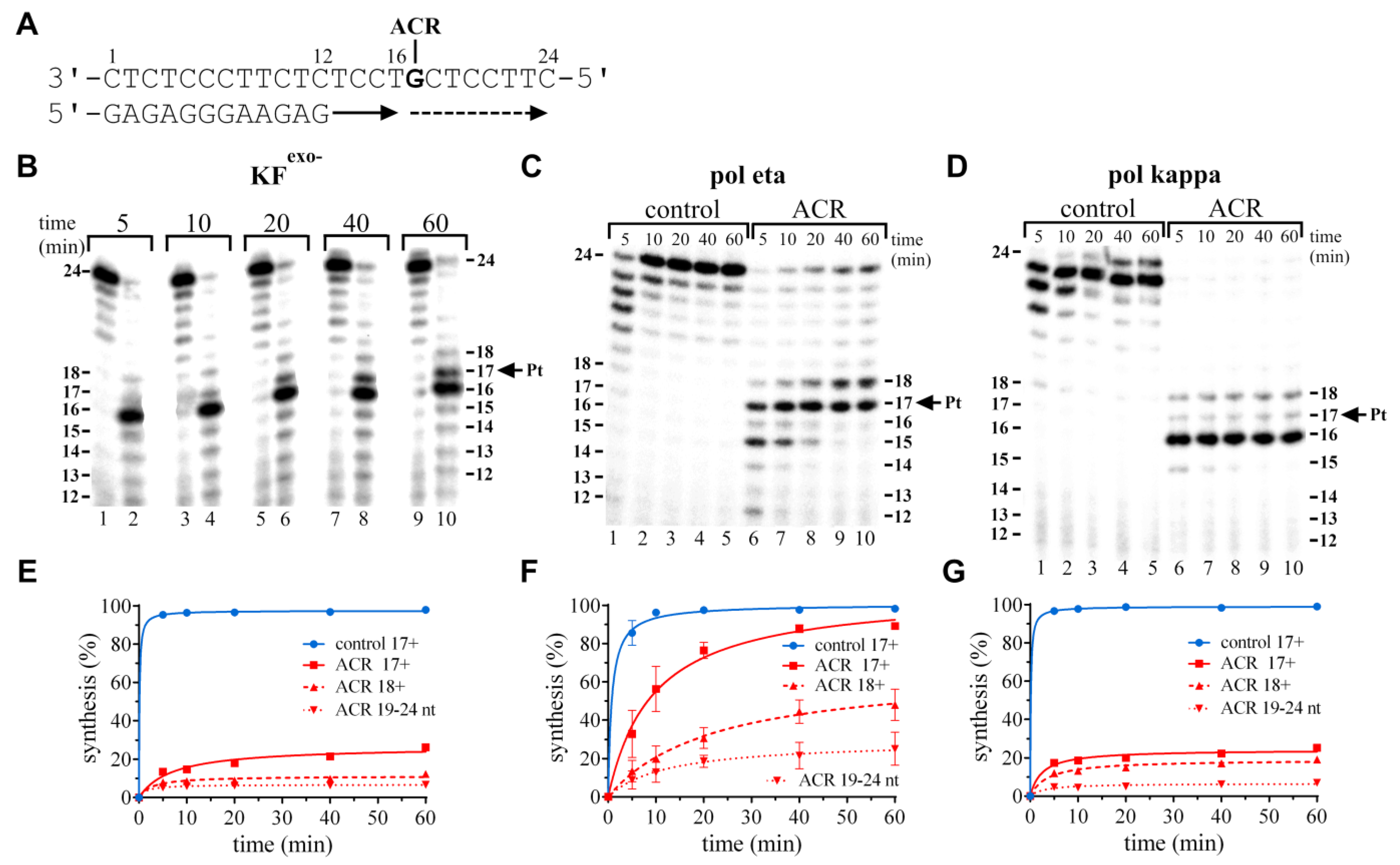
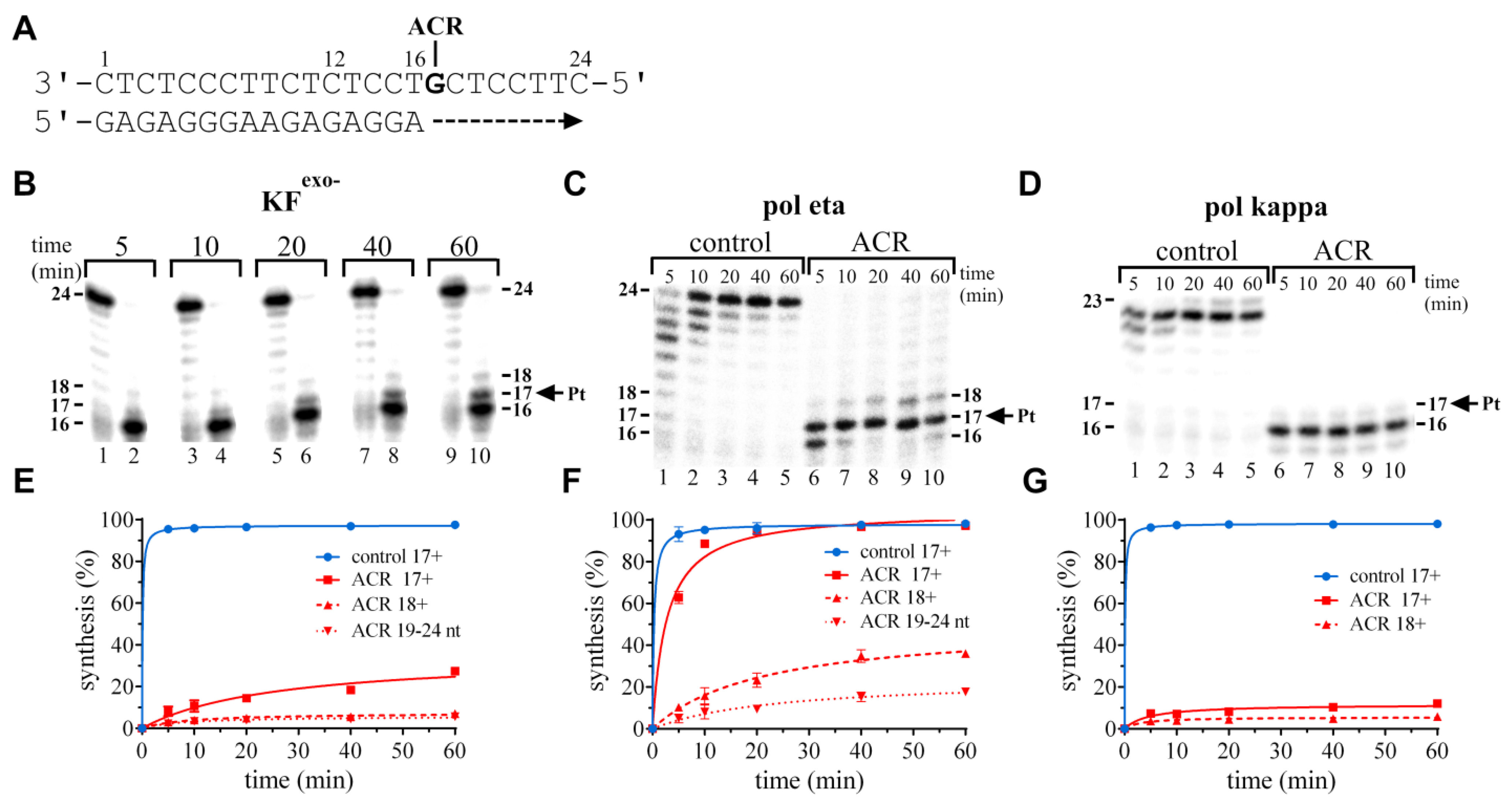
Ijms Free Full Text Processing And Bypass Of A Site Specific Dna Adduct Of The Cytotoxic Platinum Acridinylthiourea Conjugate By Polymerases Involved In Dna Repair Biochemical And Thermodynamic Aspects Html

Design And Applications Of Metallo Vesicular Structures Using Inorganic Organic Hybrids Sciencedirect

Density Functional And Global Optimization Study Of Copper Tin Core Shell Clusters

Octet Rule Detailed Explanation With Examples Exceptions

6 4 Ionic Bonding Chemical Bonding Siyavula
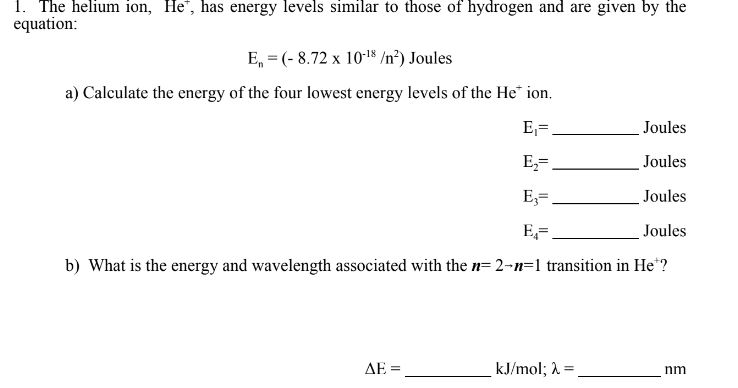
De0lkmy7z91rom

Ionic Bonding Year 12 Btec Teaching Resources
How To Know If A Bond Is Math Sp 3 Math Math Sp 2 Math Or Math Sp Math Quora

Density Functional And Global Optimization Study Of Copper Tin Core Shell Clusters

The Laws Of Thermodynamics 1st 2nd And 3rd Chad S Prep

Calculate The Percentage Ionic Character Of Si H Bond In Sih4 Assuming Pauling Electronegativity Values Of Si And H To Be 1 8 And 2 1 Respectively